2024
07-31
ARMI series launches in the US market - Jiyuan Medical accelerates its overseas expansion
Disposable electronic endoscopes have emerged
Compared to CCD (Charge Coupled Device) image sensors, CMOS (Complementary Metal Oxide Semiconductor) image sensors have the characteristics of small size, low cost, and fast processing speed. They can achieve modularization of electronic lenses and image sensors, leading to the emergence of disposable endoscopes. Since 2016, disposable electronic endoscopes have gradually become the most shining star in the global minimally invasive surgical equipment (MISE) market!
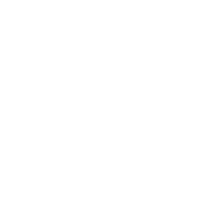
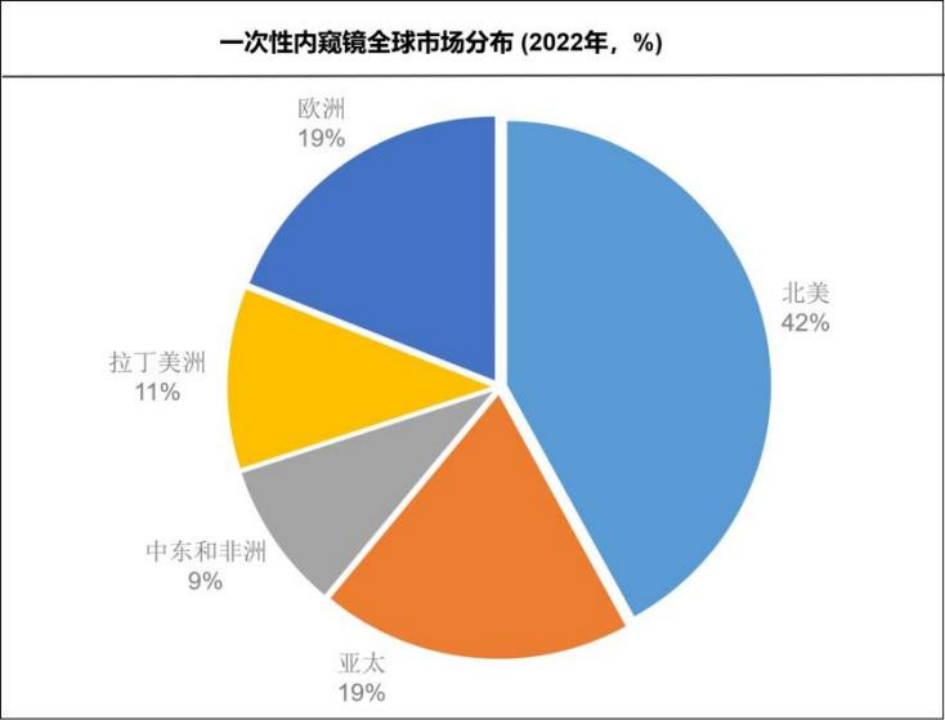
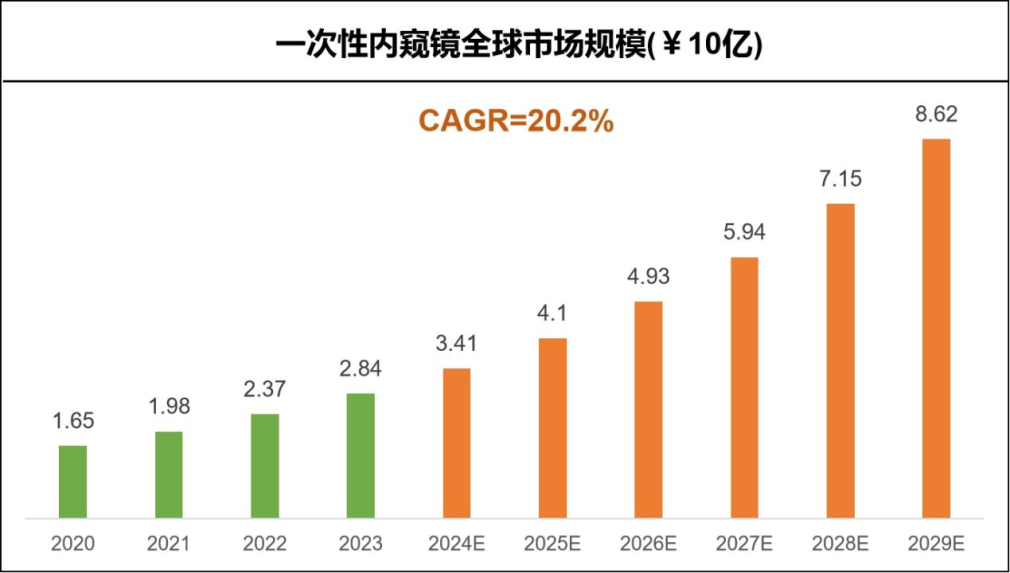
According to Precedence Research, the global disposable endoscope market has reached $2.84 billion in 2023 and is expected to reach $10.04 billion by 2029, with a compound annual growth rate (CAGR) of 20.2%.
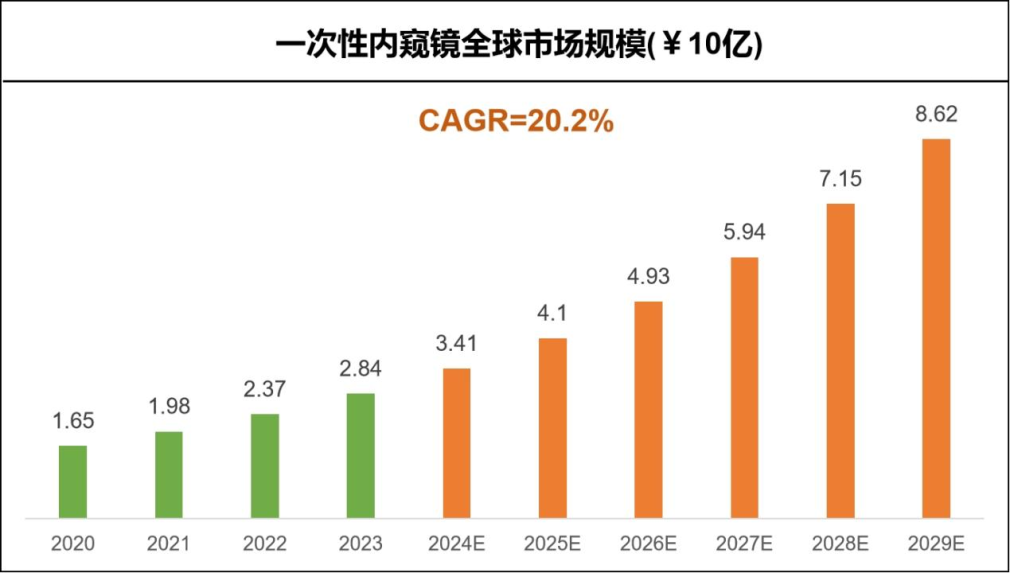
Among them, North America, Europe, and the Asia Pacific region have the largest market size for disposable endoscopes, accounting for 42%, 19%, and 19% respectively.
The Only Way to Go Overseas: Seizing the American Market
As the leader in the North American market, the United States has a disposable endoscope market size of $523 million by 2023, accounting for 18.4% of the global disposable endoscope market; By 2029, it can reach $2.044 billion with a market share of 20.3%.
In 2016, the world's first disposable electronic hysteroscope was approved by the US FDA. Due to issues such as cost, technological maturity, and charging codes, disposable electronic hysteroscopes did not truly enter the US and global markets until 2019. Subsequently, it suffered from a three-year epidemic until 2023, when disposable electronic hysteroscopes emerged in the global market.
Embark on the 3A+era of global hysteroscopy diagnosis and treatment
Jiangsu Jiyuan Medical Technology Co., Ltd. (hereinafter referred to as "Jiyuan Medical") was established in 2017, focusing on the research and development, production, and sales of disposable gynecological endoscopes, and committed to creating a "comprehensive solution for disposable gynecological endoscopes". Led by disposable electronic hysteroscopes, we provide a series of disposable electronic hysteroscopes and matching disposable surgical instruments that meet the needs of all surgical scenarios for uterine diseases. Through independent innovation and optimization integration, establish a multi product technology platform for disposable endoscopes, and create a comprehensive solution for disposable gynecological endoscopes that conforms to the 3A+gynecological endoscopic diagnosis and treatment concept and the "five in one" approach. Global synchronous registration and certification, promoting the diagnosis and treatment concept of Natural Orifice Transluminal Endoscopic Surgery (NOTEs), is leading the new era of disposable gynecological endoscopes 3A+.
3A+is the first endoscopic diagnosis and treatment technology concept proposed by Jiyuan Medical in the world. Jiyuan Medical has always focused on this concept in research and development, manufacturing, marketing, and medical services. 3A+specifically refers to "anytime, anywhere, anyone+No Cross Infection Risk".
ARMI independently developed, produced, and sold by Jiyuan Medical ® The disposable electronic hysteroscope series has passed registration and certification in more than 20 countries and regions, including China NMPA, the United States FDA, and the European Union CE-MDR. Its sales network covers more than 40 countries and regions including China, Southeast Asia, the Middle East, the United States, and Europe. Jiyuan Medical passed the ISO13485 quality system certification in November 2021. The ARMI ® series uses medical grade chips, modularization, and semi-automatic batch production. After a multi center clinical control study, and ten thousand cases of use in thousands of hospitals around the world, its product quality, functional performance, quality consistency, and technological progressiveness have reached the international leading level, so it has won unanimous praise from thousands of doctors around the world.
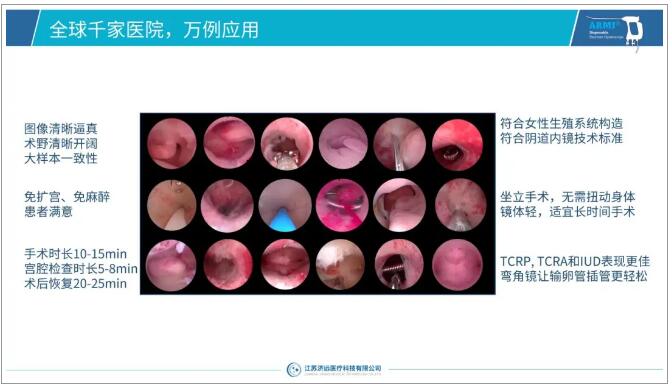
全球千院万例应用总结
历经6年的精耕细作,ARMI在注册准入、产品质量和市场覆盖等诸多方面均取得业内领先地位,正昂首挺胸向“中国第一、世界领先”的市场目标稳步迈进!
ARMI一次性电子宫腔镜登陆美国
2023年3月,济远医疗开始在美国建立“一次性电子宫腔镜示范培训中心”。经过多家示范培训中心、为期5个月的广泛试用和对照评价验证:ARMI®一次性电子宫腔镜在整机性能、局部功能及配套完整性等诸多方面,均显著优于对照品牌。
ARMI®一次性电子宫腔镜具有电子化程度高、镜体细(<5.0mm)并配置5Fr-6Fr器械通道、循环灌注等特点,符合宫腔镜阴道内镜技术准入要求,符合“即约即诊、即诊即治”的门诊宫腔镜诊疗理念。采用一次性电子宫腔镜配套“钳夹”技术的一次性内窥镜手术器械,开展“五免”(免窥器、免宫探、免扩宫、免夹持和免麻醉)宫腔镜诊疗术,将最大程度提高诊疗效率,减少宫颈损伤而保护生育力,能真正避免交叉感染的风险;尤其适用于子宫异常、传统复用式宫腔镜失败、特殊疾病和未有性生活史等患者。因此,一次性电子宫腔镜将成为全球妇科门诊和日间手术的基石。
ARMI®全球医学顾问团一致宣称:“济远医疗正在全球掀起一场重要的医学革命(An important medical revolution is unfolding globally by Jiyuan Medical)”。Dr. Resad Paya Pasic对ARMI®品牌进行了高度诠释“Advanced Medical Research Institute”,并将其推荐给美国M上市公司。
M公司长期专注于开发、制造和商业化微创解决方案,以满足女性子宫保健需求。M公司已经建立广泛商业化和微创子宫切除术的产品线,在异常子宫出血(AUB)诊疗领域已取得领先优势。2023年,M公司年妇科微创手术器械销售收入已达数亿美元。
双方就ARMI®一次性电子宫腔镜的生产、质量管理、市场策略和服务支持进行了数次的交流及论证。2024年1月,M公司派出代表团赴济远医疗进一步探讨合作事项,并进行现场核查论证。期间,双方就8类82项问题逐一进行了现场检查、沟通和复核。现场论证后,M公司代表团对济远医疗的生产、质量、研发、营销、服务支持等体系进行了高度评价:“完全符合美国FDA标准要求,已达到国际领先水平”。
2024年3月6日,济远医疗与M公司就ARMI®一次性电子宫腔镜在美国市场的销售、市场推广和服务支持签订合作协议,并就未来共同研发、生产和销售微创手术器械达成战略合作意向。

US delegation visits medical site in Jiyuan for inspection
Leading the era of disposable electronic hysteroscope 3A+
Against the backdrop of increasingly fierce competition in the global medical equipment market, sustained technological innovation and market sensitivity are key to the survival and development of enterprises. ARMI January 2024 ® The second generation of disposable electronic hysteroscope series, with a total of 60 specifications, has been registered and certified by NMPA.
The second generation of ARMI not only inherits the functional and performance advantages of the first generation, but also has lower costs; It can quickly enter the high-end markets of developed countries such as Europe and America, and also adapt to the mid to low end markets of Southeast Asia, Africa, and other countries; While reducing the cost of medical treatment for patients, it also further enhances the allocation of primary healthcare resources and service capabilities. The second generation of ARMI adopts high-definition resolution chip modules and is equipped with high-performance image processors, greatly improving the data processing capability and signal processing level in complex uterine cavity environments. It can effectively identify and diagnose hidden lesions, reduce missed diagnosis or accidents.
ARMI ® Disposable Electronic Hysteroscopy Second Generation System
At the end of March 2024, Jiyuan Medical has completed the production and delivery of its first batch of products, marking ARMI ® Disposable electronic hysteroscope officially enters the US market, benefiting 6 million intrauterine disease patients in the United States! At the same time, in the first quarter of 2024, ARMI ® disposable electronic hysteroscopes have been registered and certified in nearly 10 countries, including UKCA in the UK.
Looking ahead to 2024, the ARMI ® disposable electronic hysteroscope series will complete registration and certification in over 130 countries and regions, with sales networks covering more than 90 countries and regions, establishing its market position as "China's first and world leading", leading the global hysteroscopy diagnosis and treatment 3A+new era, and benefiting millions of patients with uterine diseases worldwide.
Zhang Yunfei, Chairman of Jiyuan Medical, said, "ARMI ® The launch of disposable electronic hysteroscopes in the United States and their entry into the high-end North American market is a manifestation of Jiyuan Medical's value concept of "quality first, professional focus". The US market is a bottleneck and difficulty for many domestic enterprises to go global, and entering the US market is a major leap forward for Jiyuan Medical's globalization strategy! We firmly believe that ARMI ® will gradually become an internationally renowned brand, leading the era of global popularization of hysteroscopy diagnosis and treatment.