R&D route
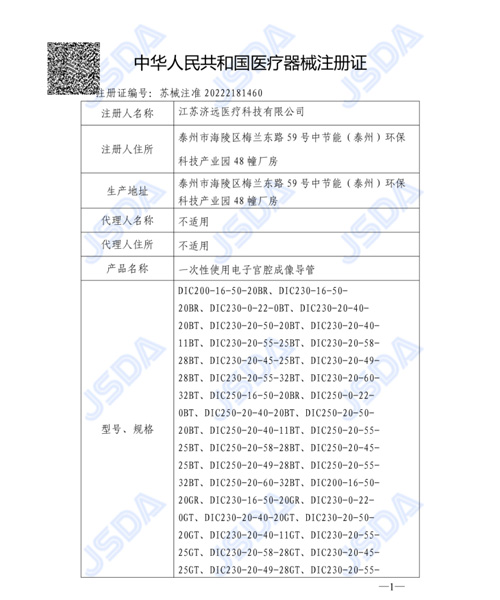
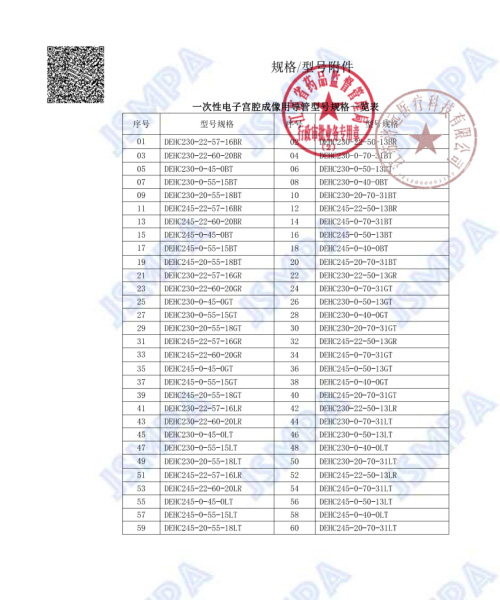
Since its establishment, the company has clearly focused on gynecology and gradually formed a comprehensive solution for gynecological endoscopy based on disposable electronic hysteroscopes, becoming a global leader in disposable hysteroscopes. At present, we have successfully obtained China NMPA certification, US FDA certification, EU 13485 certification, and EU MDR certification for multiple products such as hysteroscopy image processor systems, disposable electronic hysteroscopes, and matching disposable operating instruments. This move not only fills the gap in the domestic market for disposable gynecological endoscopes, but also makes the company the first in the world to obtain certification from China, the United States, and Europe and achieve mass production in the field of disposable hysteroscopes. In the future, enterprises will continue to deeply cultivate in the field of gynecological medical devices, conduct in-depth research and development to solve clinical pain points, continuously reduce clinical usage costs, and enable more patients to "look up to" and "look at it well".